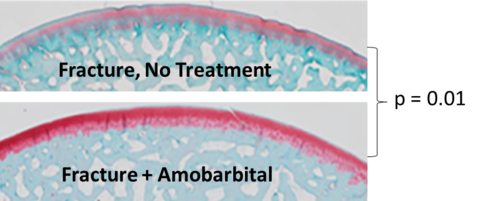
Sustained efforts by a multidisciplinary research team led by Dr. James Martin established PTOA stemming from joint trauma as a mitochondrial disease arising from mechanical insult to cartilage, a process that proved to be subject to intervention with small molecule drugs (https://doi.org/10.1002/jor.23389). One such drug, amobarbital, was tested in a clinically realistic Yucatan minipig model of PTOA induced by joint fracture developed in our labs under the leadership of Dr. Jessica Goetz (https://doi.org/10.1016/j.joca.2015.05.022). A study led by Drs. Mitchell Coleman and Martin in our group showed that a single amobarbital dose administered by intra-articular injection soon after fracture dramatically slowed cartilage degeneration, a hallmark of PTOA progression (Figure 1) https://doi.org/10.1126/scitranslmed.aan5372).
The chondroprotection afforded by amobarbital in these pre-clinical studies formed the basis for DoD funding of a clinical trial to investigate the safety of the drug and its effects on the risk for PTOA in people with ankle fractures. Complimentary toxicology studies in Yucatan minipig joints showed that doses of up to 50 times higher than the therapeutic dose were well-tolerated, indicating a broad margin of safety. These findings lent strong support for an investigational new drug submission to the FDA, which was approved by the agency in July 2021. IRB approval to recruit six patients for phase I is expected in early 2022. Provided the dose proves to be well-tolerated in three-month follow-ups, an additional 60 patients will be recruited for phase II. Given the rapid progression of PTOA in fractured ankles, the two-year follow-ups in this phase are expected to provide preliminary evidence for efficacy that will support more definitive investigation.